thanks Pfizer, for your pfabulous and pfun report
by Jessica Rose, December 5th 2021
5.3.6 CUMULATIVE ANALYSIS OF POST-AUTHORIZATION ADVERSE EVENT REPORTS OF PF-07302048 (BNT162B2) RECEIVED THROUGH 28-FEB-2021
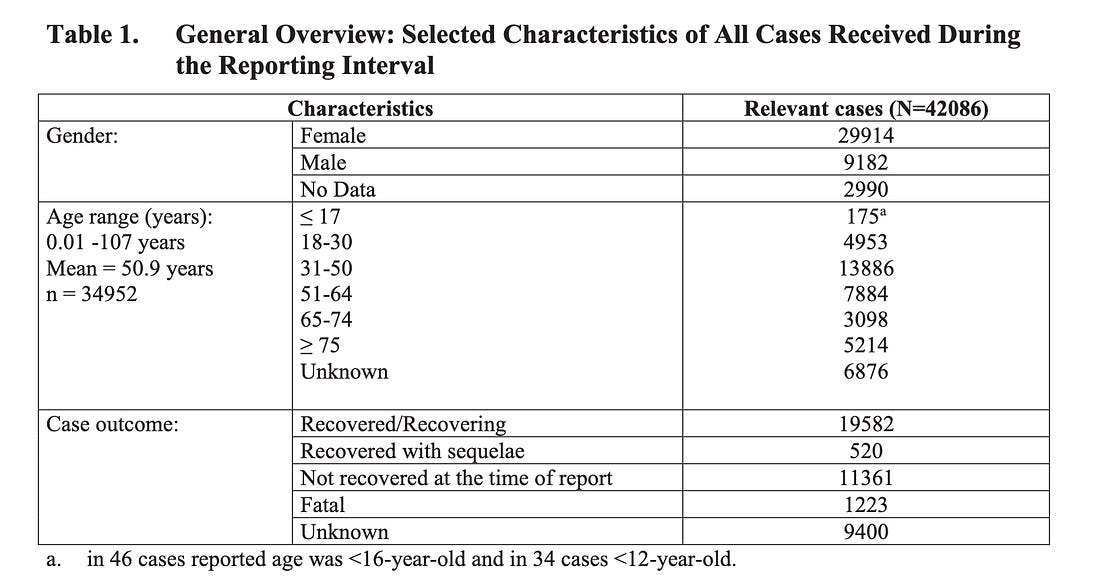
And here’s the first summary table.
So the first thing we notice is the date. These adverse event reports are from the start date of the roll out into the U.S. population on December 17th, 2020 up to and including February 28th, 2021. That’s 74 days or 2 months and 12 days.
The second thing we notice is the total adverse event count of N = 42,086. Now, if you count the number of adverse events reported to VAERS for this exact time frame according to injection dates, you get N = 84,770 reports in the context of the Pfizer/BioNTech products. It’s literally twice the N reported by Pfizer in their report. Either way, you’re facing 42,086 or 84,770 reports of adverse events in 74 days. And what of the under-reporting factor? I will come back to that…
The third thing we notice is the high number of adverse event reports for children. There are 34 adverse event reports made for children less than 12. How is that possible? Children this young weren’t supposed to be injected in the early days since there was definitely no safety data accumulated at that point. The exclusion criteria age cut-off was 12 in Pfizer’s phase III trial, so how is this possible? And how many of these very young children died?
UPDATE: Monday December 6th, 2021
Baby details. So let’s head down to page 15 in the document where you’ll find a table called ‘Description of Missing Information’. In the descriptive subtext underneath, go to letters ‘c’ and ‘d’.
c. Upon review, 31 additional cases were excluded from the analysis as the data reported (e.g. clinical
details, height, weight, etc.) were not consistent with paediatric subjects
d. Upon review, 28 additional cases were excluded from the analysis as the data reported (e.g. clinical
details, height, weight, etc.) were not consistent with paediatric subjects
Paediatric subjects are people less than 12 years of age. Now correct me if I am wrong here but does this not state that a total of 59 reports of adverse events were dropped because the paediatric subjects (babies) did not fit the height and weight criteria? What the hell does ‘etc.’ entail? Shouldn’t, oh, I don’t know, ‘age’ be one of the criteria used to justify dropping a paediatric case from the analysis if specific parameters are being used as reasons to drop them? To me, it is NOT unreasonable to assume that some of the children in the paediatric cohort were not <12, so this would actually be a reasonable reason to drop them from this cohort and analysis of paediatric subjects. But we have to assume that this is not the case and that all paediatric subjects were <12 years of age and that 59 of them out of 132 (45%) (page 13) were dropped because they were too tall or too fat.
By the way, it is not uncommon to see paediatric VAERS reports ‘disappear’. Is this the weak justification that is being used? They were too fat? Too tall? They couldn’t possibly be a baby. Oh my word.
The fourth thing we notice is the word ‘Fatal’. Of all of the 9 pages of adverse events listed at the end of this document, that one is permanent. Beside this word, we see the number 1,223. 1,223 people died within 74 days of taking the Pfizer shot. That’s more than 0, right? 1,223. Wow. That’s almost 25 times the number of peope who were allowed to die historically, before a previous vaccine was withdrawn for having been deemed ‘unsafe’.
How many people had died in this time frame that had filed reports to VAERS? Well, it depends on whether or not you count spontaneous abortions as deaths, actually. I do count them as deaths. It also depends on the date data that you use to count.
The number of deaths reported to VAERS in the context of the Pfizer/BioNTech injectable products between December 17th, 2020 and February 28th, 2021, can be calculated using the injection dates, the onset dates or the death dates. The table below summarizes the total death counts, with and without miscarriages, for each representative date type. The minimum count, 874, is obtained using the DATEDIED column vector and the maximum count, 2,145, is obtained using the VAX_DATE column vector.
VAX_DATE ONSET_DATE DATEDIED
w/miscarriages 2145 1194 874 w/o miscarriages 1954 1073 874 difference 191 121 0However, you slice it, lots of people died and filed reports to VAERS in the context of the Pfizer product. So we have a confirmation of over a thousand dead people in Pfizer’s own report on our hands.
The fifth thing we notice can be found in Table 6 on page 15 where you’ll find the table called ‘Description of Missing Information’. Again. In the descriptive subtext underneath, go to letter ‘b’.
b. 558 additional cases retrieved in this dataset were excluded from the analysis; upon review, 546 cases cannot be considered true lack of efficacy cases because the PT Drug ineffective was coded but the subjects developed SARS-CoV-2 infection during the early days from the first dose (days 1 – 13); the vaccine has not had sufficient time to stimulate the immune system and, consequently, the development of a vaccine preventable disease during this time is not considered a potential lack of effect of the vaccine;
This section ‘b’ comment on excluded data pertains to ‘Lack of efficacy cases’. So they dropped 558 people who developed SARS-CoV-2 infection following injection. When someone succumbs to a breakthrough infection, this is referred to as ‘lack of efficacy’. Some people would call it Vaccine Failure. The justification was this: 546 of these people got COVID-19 within 14 days of the injection because their immune systems didn’t have time to work. And therefore, any SARS-CoV-2 infection that came about during this time couldn’t possibly be due to the inefficacy of the product. It’s the immune system’s fault. It’s definitely not the uselessness of the product.
You know. I could even have gotten behind an argument that claimed that perhaps they already had been exposed to SARS-CoV-2 and that is both why they ‘developed SARS-CoV-2 infection during the early days’ and also why they should be excluded from the analysis. But this is not what they wrote here. They wrote that these people were dosed, developed SARS-CoV-2 (whatever the hell that means) within 13 days, were APPROPRIATELY coded with MedDRA code ‘Drug ineffective’, and then removed from the analysis because ‘the development of a vaccine preventable disease during this time is not considered a potential lack of effect of the vaccine’. So, you have to wait for day 14 to hit before you can truly say that your COVID-19 is due to the ineffectiveness of your shots.
…the PT Drug ineffective was coded but…
There’s no ‘but’ that belongs here, in my opinion. They were coded this way because they got COVID-19 anyway and within 2 weeks of shot. The immune system isn’t some sluggish, rusty machine. It responds to foreign proteins/pathogens with extreme rapidity and effectiveness in people who are not immune-compromised or full of senescent cells. Their argument is lame and obtuse and based on semantics, in my opinion. It sounds very much like a lawyer wrote these words; no capable immunologist or drug efficacy assessor would write that.
The sixth thing we notice is shocking and related to miscarriages that were bumped from the analysis. This data can be found in Table 6 on page 12 where you’ll find the table called ‘Description of Missing Information’. Again.
Pregnancy cases: 274 cases including:
– 270 mother cases and 4 foetus/baby cases representing 270 unique pregnancies (the 4 foetus/baby cases were linked to 3 mother cases; 1 mother case involved twins)
– Pregnancy outcomes for the 270 pregnancies were reported as spontaneous abortion (23), outcome pending (5), premature birth with neonatal death, spontaneous abortion with intrauterine death (2 each), spontaneous abortion with neonatal death, and normal outcome (1 each). No outcome was provided for 238 pregnancies (note that 2 different outcomes were reported for each twin, and both were counted)
Now. Really try to understand what they wrote here. 274 women who were reported to have been injected while pregnant (‘Use in Pregnancy and lactation’) were dropped from the analysis. 270 of these pregancies were considered unique cases. 23 of these women (8.5%) suffered miscarriages. But wait. At the bottom they write that there’s no data for 238 of these pregnancies. 23+5+2+2+1+1=34. There are 34 reports where outcomes were available. 238+34=272. So, the actual number of data points liked to an outcome is 34. So this means that 68% of the women suffered a miscarriage. This is completely in-line with the study in the NEJM that claimed safety in the pregancy context that was subsequently forced to publicly correct a mistake based on an inappropriate denominator. This correction changed the reporting frequency of miscarriages in test subjects in the first and second trimesters from 12.6% to 83%. This is documented.
Now let’s move to pages 8 and 9 where you can find a table of Adverse Events with absolute counts and percentages. This table shows the most commonly (≥2%) reported MedDRA (v. 23.1) PTs in the overall dataset (through 28 February 2021). In the table below, I match the VAERS numbers by Adverse Event MedDRA code to compare them with the Pfizer numbers.
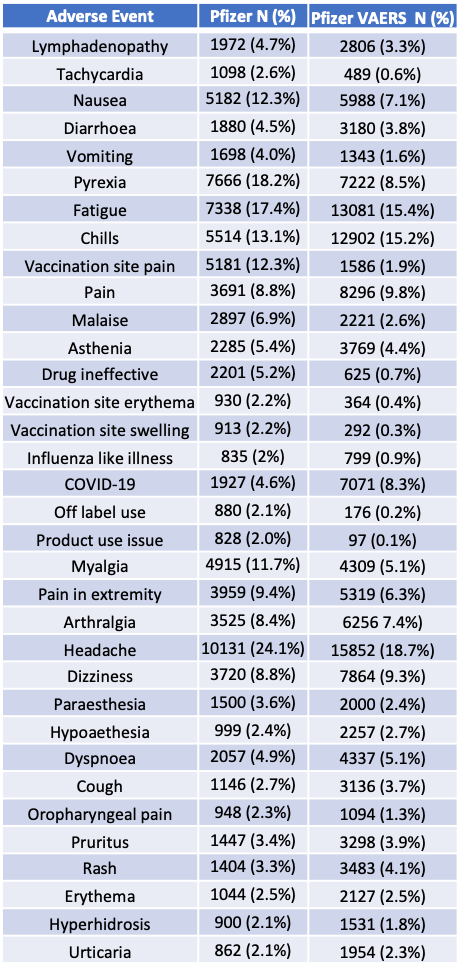
The first problem I have with this is not the mismatch of the counts – it’s the lack of AEs that are sending blaringly loud safety signals from VAERS like Myocarditis. Where is it? Tachycardia is the only AE reported in the realm of cardiovascular issues? Really? Where’s Death? Where’s Bell’s Palsy? Where’s Herpes Zoster? All the AEs reported above 2%? Really. No my pfizer pfriends. You are not being transparent.
To be continued… on a call
Source: jessicar.substack.com/p/pfizer-adverse-event-data