The U.S. Food and Drug Administration published a report to justify authorizing COVID-19 vaccines for infants and toddlers, but the report is just more of the same: exaggerating the risk of the virus and minimizing the perception of the risk posed by the vaccines.

As promised, the U.S. Food and Drug Administration (FDA) has ginned up a report that ostensibly will be used to try to justify “approval” (whatever they mean by that now) of COVID-19 vaccines for infants and toddlers (children < 5 years old).
This report comes after a torrent of massive reports from Moderna and Pfizer that claim to review studies of the safety and efficacy of COVID-19 vaccines in children.
It is not hard to see what shenanigans the FDA has been up to — to try to bolster a vaccine that fewer and fewer adults want. It’s more of the same: exaggerating the apparent risk of the virus and minimizing the perception of risk posed by the vaccines.
In other words, lies.
1. There is no evidence of clinical urgency.
Infants and toddlers (and children in general) do not get COVID-19; they do not (yet) die from COVID-19.
All that can change when antibody-dependent enhancement kicks in for the vaccinated.
The FDA’s own reports cite 1,086 deaths “from COVID-19” and 10,700,000 “cases” of COVID-19 in children aged 0-17. There have been 832 days since April 1, 2020, when diagnoses started for COVID-19.
For the entire population of children in the U.S. (73,000,000), the risk of COVID-19 infection since the onset of COVID is 10,700,000/73,000,000 = 0.14657.
The risk of a child dying if they have a diagnosis is 1,086/10,700,00 or 1086/10700000 = 0.00010149532. The risk of any child dying of COVID-19 over this time period is 1,086/73000000 = 0.00001487671.
The per-day risk is on the order of 1.78806611e-8 (0.000000001788). There is no real unmet clinical need and the FDA needs to go back to college to understand how to use RT-PCR correctly. Children do not get COVID-19, and they do not die.
2. Inconsistent use of the idea ‘vaccinated.’
This has been the pattern from the very first study. The FDA, the Centers for Disease Control and Prevention, Moderna, Pfizer and others pull out whatever definition of “vaccinated” they want.
Examples: “Vaccinated” is defined in the original trials as people who received both doses and who did not develop COVID-19 before two weeks passed after the second exposure to the vaccine.
In fact, that means that people who developed COVID-19 due to disease enhancement were dropped from the study calculations.
First, this is the first time people were dropped from a vaccine trial for getting infected with the pathogen targeted by the vaccine up to 13 or 14 days after being vaccinated.
Second, it’s actually five entire weeks — one month and one week — 44 days — after the first exposure. ALL of the vaccine efficacy being cited by the FDA is suspect.
Moderna’s and Pfizer’s vaccines never achieved >90% true vaccine efficacy; the best estimate is more like 75%.
3. Inconsistent use of the idea ‘vaccine efficacy.’
Over the time period since the first COVID-19 vaccine trials, various definitions of “vaccine efficacy” have been used.
Decreased transmission. Reduction in infection rates. Reduced hospitalization. Presence of neutralizing antibodies. Presence of antibodies.
All are used and cited in the FDA’s report whenever convenient, all in an ad-hoc manner. It’s more than irritating. It’s moving the goal post and represents reckless (and ineffective) attempts to manipulate public perception.
This practice continues in the untrustworthy reports and studies that are cited by the FDA.
Further evidence of the futility of the evidence used to claim efficacy comes from Moderna’s Sponsor Briefing report to the FDA:
“3.3 Regulatory Considerations for Clinical Development of COVID-19 Vaccines in Children
“Effectiveness…
“Regulatory precedent with other preventive vaccines provides a basis for inference of vaccine effectiveness in pediatric populations based on immunobridging to a young adult population in which clinical disease endpoint vaccine efficacy has been demonstrated for the same prototype vaccine. The immune marker(s) used for immunobridging do not need to be scientifically established to predict protection but should be clinically relevant to the disease.
“Based on available data in humans and animal models, FDA considers neutralizing antibody titers (a functional measure of the vaccine immune response against SARS-CoV-2) to be clinically relevant for immunobridging to infer effectiveness of COVID-19 vaccines in pediatric age groups.
“Because no specific neutralizing antibody titer has been established to predict protection against COVID-19, two immunogenicity endpoints (GMT and SRR) are considered appropriate for comparing the range of neutralizing antibody responses elicited by the vaccine in pediatric versus young adult populations.”
Also embedded in this piece of work is the fact that the FDA does not need evidence of long-term immunity; they are settling for something called “immunobridging” — guessing at the efficacy of a vaccine in one clinical population from measurements made from other clinical populations.
They also are making people dependent on vaccines … expecting patients to have antibodies from one vaccine to the next. This makes no sense immunologically. We don’t need continuously high antibody levels against any pathogen.
We have memory B-cells and T-cells. In accepting this paradigm, the FDA is completely off its rocker and will cause immune exhaustion with constant vaccinations every three to four months.
4. Incomplete consideration of the scientific data (Barnstable County, Israel, Ontario).
We know that months after vaccination, those who are vaccinated are at higher risk of infection and now of hospitalizations.
According to Jeremy Hammond, data show “vaccine effectiveness (VE) in children becomes(sic) negative within several months since receipt of the second dose.in children.”
Hammond wrote:
“Researchers from the New York State Department of Health published a study on the preprint server medRxiv on February 28 noting that the evidence for vaccine effectiveness in children, particularly those aged five to eleven, was ‘limited.’
“So, they aimed to provide data to inform policymaking.
“‘During Omicron variant predominance,’ the authors concluded, ‘VE against infection declined rapidly’ for young children in the state of New York, ‘with low protection by one month following full-vaccination.’
“Comparing COVID-19 cases during January between unvaccinated and vaccinated children, they estimated initial vaccine effectiveness for children aged twelve to seventeen to be 76 percent, but this dropped to below 50 percent after just five weeks since receipt of the second dose.
“Moreover, for young children (aged five to eleven), they observed a drop from 65 percent to just 12 percent after only one month.
“Thereafter, their estimate indicated significantly negative effectiveness for this age group, as shown in Figure 2 of their paper: by 35 to 41 days, VE reached negative 10 percent, and by 42 to 48 days, it reached negative 41 percent.”
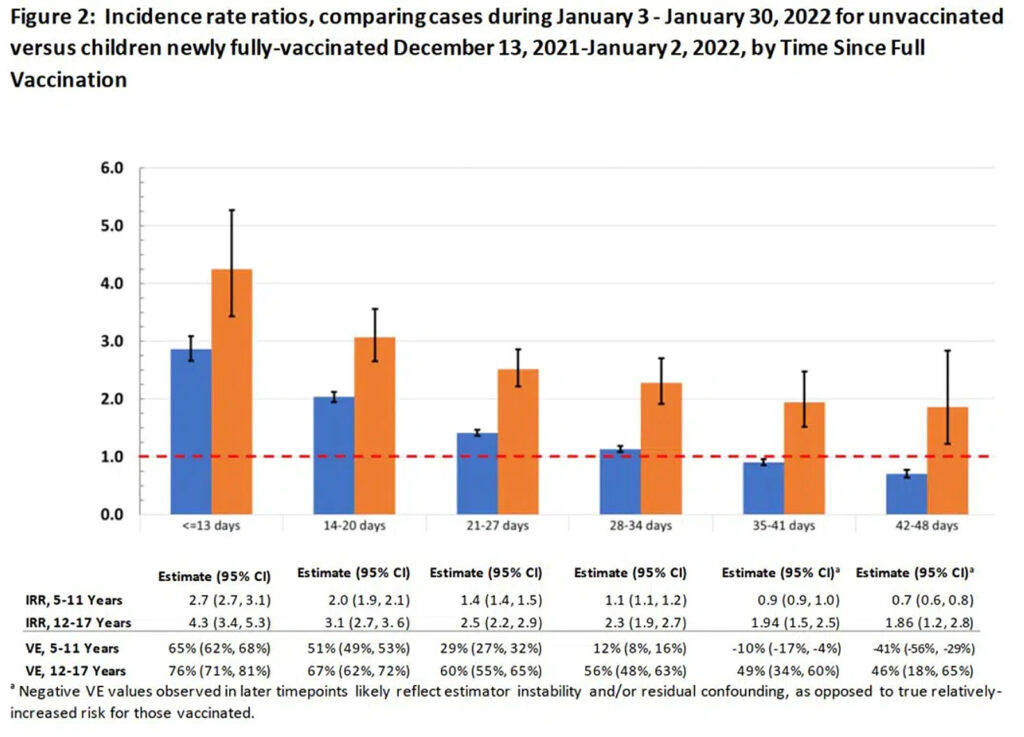
Jeremy goes on to report (correctly) that the authors of the article misinterpreted their own data.
5. Moderna and Pfizer reports fail to study long-term risks.
In this report, for example, Moderna offers data on myocarditis only up to day 28 after the vaccine.
Why day 28? Why not “since the vaccine has been administered” to more accurately reflect the real-world clinical situation?
They also state that myocarditis is a large concern in people infected with SARS-CoV-2 — but the comparison is to the uninfected, not the vaccinated, and we know that the spike protein is the cause (syncytia among heart muscles caused by the spike protein).
The spike protein, of course, is the basis of their mRNA vaccines.
6. Incestuous conflicts of interest/unjustified influence by regulators.
Peter Marks, M.D., Ph.D., is charged with setting the decisions at the FDA on whether to consider vaccines for specific populations.
That “study” is also guilty of all of the same loose logic as above; it is noteworthy that the study assumes a “worst-case scenario” of zero deaths from myocarditis following COVID-19 vaccination.
Credit: Toby McDonald, who wrote this:
“I’m reading the Moderna “Sponsor Briefing Document” and they built their benefit-risk assessment off of Funk et al. (2022). So I looked up Funk and it’s a recent paper by six staffers at the FDA including Peter Marks, Richard Forshee, and Hong Yang (who wrote the dreadful benefit-risk assessment for kids 5 to 11 back in October). Quite literally in their “worst-case scenario,” they predict 0 deaths from myocarditis in the vaccine group. It’s a stunning work of fiction.”
Source: childrenshealthdefense.org/defender/fda-report-covid-shots-infants-toddlers/?utm_source=salsa&eType=EmailBlastContent&eId=fee61304-a3e2-40e3-b46f-9adae2dcd863
